中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 彭娟娟, 李向敏, 王涓, 谢意珍, 吴清平. 2021
- Juanjuan Peng, Xiangmin Li, Juan Wang, Yizhen Xie, Qingping Wu. 2021
- 食药用真菌多糖对肿瘤免疫逃逸调节作用机制研究进展
- Research progress on regulating tumor immune escape mechanism of edible and medicinal fungal polysaccharides
- 微生物学报, 61(9): 2594-2606
- Acta Microbiologica Sinica, 61(9): 2594-2606
-
文章历史
- 收稿日期:2020-10-13
- 修回日期:2020-11-03
- 网络出版日期:2020-11-13
2. 广东省科学院微生物研究所, 华南应用微生物国家重点实验室, 广东省微生物安全与健康重点实验室, 广东 广州 510070
2. State Key Laboratory of Applied Microbiology Southern China, Guangdong Provincial Key Laboratory of Microbial Safety and Health, Institute of Microbiology, Guangdong Academy of Sciences, Guangzhou 510070, Guangdong Province, China
癌症是严重威胁人类生命和健康的重大疾病,由于癌细胞来源于自体细胞的基因突变,具有强的遗传不稳定性和异质性,故放化疗及手术在大多数情况不能完全清除肿瘤细胞,且易导致耐药性以及更难治疗的肿瘤复发。基于肿瘤学和免疫学的深入发展和理解,研究发现肿瘤的形成是机体免疫逃逸的结果,利用免疫治疗法逆转免疫逃逸恢复机体免疫识别和清除肿瘤细胞的能力成为研究热门[1]。
食药用菌作为传统中药治疗药物,具有调节人体免疫力和抑制肿瘤生长的功能,其活性成分包括多糖[2]、萜类物质[3]、麦角甾醇[4]、有机酸和酚类等。我们团队前期研究中发现食药用菌萜类及甾醇类物质有效地抑制肿瘤增殖、迁移和侵染,具有明显的抗肿瘤作用[5],并发现多糖主要通过免疫调节的途径发挥抗肿瘤作用,对肿瘤免疫微环境具有明显的正向调控作用。以此为基础查阅总结出国内目前已经上市的多糖类药物,大多作为免疫增强剂整体增强机体免疫功能(表 1)。但随着对食药用真菌多糖免疫功能深入研究及肿瘤免疫技术和手段的优化,近年研究表明食药用真菌多糖可以通过调节肿瘤微环境增强微环境内T细胞对肿瘤细胞的识别能力抑制肿瘤免疫逃逸发挥抗肿瘤功效[6]。本文将对食药用真菌多糖在抑制机体肿瘤生长过程中对肿瘤细胞表面抗原、免疫细胞类型及表面受体的影响进行综述和分析,总结食药用真菌多糖作为免疫调节剂在肿瘤免疫逃逸治疗中的生物活性,探讨其抑制肿瘤功效及与肿瘤免疫的作用机制。
| Polysaccharide drugs | Dosage forms | Monosaccharide compositions | Mass average molecular weight (104 Da) | Main functions |
| Lentinan | Injection | Glucan with β-(1-3) glucose as main chain and β-(1-6) as branched chain | 50 | Improving immune function, antimour, adjuvanting chemotherapy[7–8] |
| Ganoderma sinense Polysaccharide | Tablets | α-(1-3)glucose, α-(1-4)-, (1-6) glucose | 1.94–5.90 | Improving immune function, antimour[9] |
| Polysacharidum of G. lucidum | Injection | Xylose, mannose, etc. | Improving immune function, antimour[10–11] | |
| Polyporusus Bellatus | Injection, Capsule | β-(1-3) Glucan | 50 | Adjuvanting chemotherapy, improving immune function[12] |
| Coriolus versicolor polysaccharide | Capsule | Glucan with β-(1-3), β-(1-4) or β-(1-4), β-(1-6) as the main chain with β-(1-3), β-(1-6) branched chain. Monosaccharides include glucose, galactose, mannose, xylose, etc. | 130 |
Adjuvanting chemotherapy, improving immune function[13] |
| Tremella polysaccharide | Capsule | Fucose, xylose, mannose, glucose and glucuronic acid | Anti-inflammatory, improving immune function[14] | |
| Poria cocos mushroom polysaccharides | Oral solution | Glucose, mannose, ribose, etc. | Improving immune function, antimour[15] | |
| Maitake component | Capsule, Drops | Glucan with β-(1-3) glucose and β-(1-6) glucose as branched chain | 100 | Anti-inflammatory, improving immune function[16] |
1 肿瘤免疫逃逸治疗机制研究 1.1 肿瘤免疫逃逸发生机制
肿瘤免疫逃逸是指通过各种机制避免免疫系统识别和攻击而使肿瘤细胞生长和转移的现象,这是肿瘤存活和发展的重要策略[17]。肿瘤免疫逃逸有许多诱导因素,包括肿瘤细胞的低免疫原性,肿瘤细胞通过降低自身细胞表面肿瘤特异性识别抗原的表达从而逃避免疫细胞的攻击,其次是肿瘤细胞诱导的免疫抑制。肿瘤诱导的免疫抑制以2种主要方式起作用。第一种是通过诱导免疫抑制细胞在肿瘤周围聚集并分泌免疫抑制因子而使细胞毒性T淋巴细胞(CTL)失活,从而降低免疫力。免疫抑制的第2种机制涉及诱导免疫抑制分子或其受体的表达,可以抑制效应T淋巴细胞的激活,最终导致肿瘤免疫逃逸[18]。
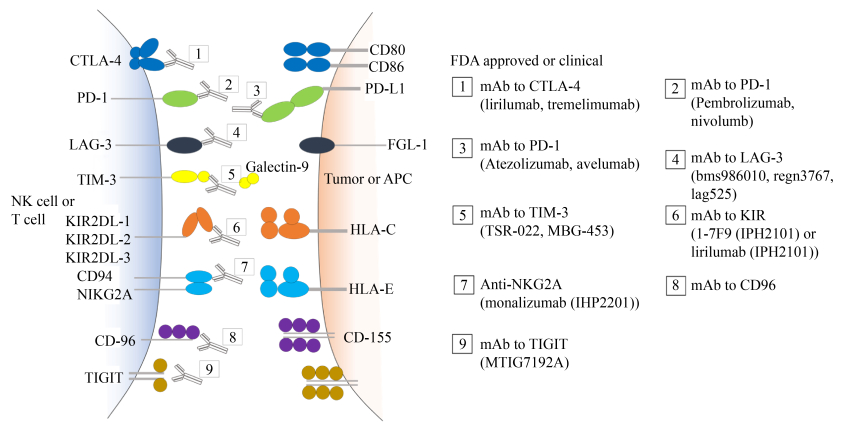
1.2 免疫逃逸治疗现状肿瘤的免疫疗法主要通过恢复免疫细胞对肿瘤细胞的识别能力以及增强其对肿瘤细胞的杀伤能力,达到清除肿瘤的目的。免疫检查点疗法作为临床上肿瘤免疫疗法的重要治疗途径,目前在肿瘤免疫治疗中取得显著效果,并获得2018年的诺贝尔生理学或医学奖,这也使肿瘤免疫治疗成为癌症治疗第四大疗法[19] (表 2,图 1所示)。但即使目前研究最清楚最有效的抗PD-1/PD-L1抗体疗法临床有效率总体上只有20%–30%[20–21]。除对免疫检查点的研究以外,临床上用到疗法还有通过改造T细胞进行嵌合抗原受体T细胞过继性免疫治疗,通过对靶向实体肿瘤的T细胞进行测试,但到目前为止仅取得有限的成果。这种治疗策略面临的困境是如何在肿瘤微环境中促进T细胞的浸润和持续作用对抗肿瘤细胞[21]。其次是通过增强抗原呈递能力、激活抗原特异性效应和记忆T细胞的杀伤效应来设计肿瘤疫苗,但这种方式依赖的是生物标志物,如果癌细胞表面固有抗原改变及肿瘤微环境发生变化,疫苗便难以发挥作用[22]。
| Immune targeted drug names | Check points | Usages | Mechanisms and effects |
| Tremelimumab | CTLA-4 | Used alone or in combination with PD-1 blockers | Specifically binding CTLA-4 and increasing the number of CD4+ lymphocytes[23] |
| Ipilimumab | CTLA-4 | Used alone or used in combination with ICOS+Ki67+CD4+ modulator or with Foxp-3, PD-1 blocker | Specifically binding CTLA-4 and increasing the number of lymphocytes, enhancing anti-tumor immune response[24–25] |
| Avelumab | PD-L1 | Used alone | Specifically binding PD-L1 and increasing the number of CD8+ lymphocytes[26]
Prolonging the life of cancer patients[27], objective response rate (ORR) reacded 31%[24] |
| Atezolizumab | PD-L1 | Used alone | Inhibiting the binding of PD-L1 to PD-1 and B7.1, restoring T cell specific immunity to tumors[28]objective response rate (ORR) reacded 19%[29–30] |
| Pembrolizumab | PD-1 | Used alone, Used in combination with paclitaxel chemotherapy drugs, Used in combination with trastuzumab | Blocking the signal pathway between PD-1 and PD-L1 and restoring the specific recognition ability of T cells. objective response rate (ORR) reacded 15.2%[31] |
| nivolumb | PD-1 | Used alone | Specifically binding PD-1, enhancing T cell function, objective response rate (ORR) reacded 35%[32] |
| bms986010, regn3767, lag525 | LAG-3 | Used alone or used in combination with PD-1 blockers | Inhibiting the negative regulation of immune checkpoint LAG-3 on T lymphocytes, preventing tumor immune escape[28, 33] |
| TSR-022, MBG-453 | TIM-3 | Used alone or used in combination with PD-1 blockers | Specifically binding TIM-3, enhancing the ability of CD8+ to specifically recognize tumor cells[28] |
| MTIG7192A | TIGIT | Used alone or used in combination with PD-1 blockers | Blocking TIGIT, enhancing the activity and degranulation level of NK and T lymphocytes, and increasing the expression of cytokines[28, 34] |
|
| 图 1 目前国内外上市/临床研究的免疫检查点治疗药物(来自药渡—医药数据信息平台) Figure 1 Current immune checkpoint therapeutic drugs currently on the market/clinical research at home and abroad. Adapted from Yaodu-medical data information platform. |
2 食药用真菌多糖对肿瘤微环境细胞表面受体调节作用
食药用真菌多糖对肿瘤治疗与现代免疫疗法有很多相通之处,如调节肿瘤细胞表面特异性识别抗原从而激活或抑制某类免疫细胞,改善肿瘤微环境逆转免疫逃逸现象,改善机体免疫状态从而抑制肿瘤的生长。
2.1 食药用真菌多糖对免疫检查点的调节作用免疫检查点PD-1在1992年被日本科学家本庶佑团队发现,并验证其属于免疫负调控因子[35],随着陈列平教授发现PD-1受体与B7-H1 (即PD-L1)并存,并首次将共信号分子引入肿瘤免疫领域,阐明这一通路在癌症免疫中的关键作用[36]。免疫检查点药物因此被发现,其主要通过阻断肿瘤细胞表面免疫下调因子与淋巴细胞之间的信号通路达到恢复机体免疫细胞对肿瘤细胞的识别功能,而食药用菌多糖可以直接通过抑制肿瘤细胞或淋巴细胞表达多种免疫负调控因子,帮助淋巴细胞识别肿瘤细胞,达到抑制肿瘤的目的。
Ina团队[37]通过研究发现香菇多糖可以下调肿瘤细胞表面PD-L1蛋白的表达水平来刺激肿瘤特异性适应性免疫应答,恢复肿瘤细胞对化疗药物的化学敏感性,延长癌症患者的生存率,这也被认为是化学免疫疗法的协同机制。我们团队研究发现灵芝孢子粉多糖可以显著降低脾脏以及肿瘤微环境中淋巴细胞免疫检查点PD-1、CTLA-4的表达,且灵芝孢子粉多糖与化疗药物紫杉醇联合使用时,显著地抑制了肿瘤微环境中免疫检查点(PD-1和Tim-3)的表达,恢复了肿瘤微环境中肿瘤浸润淋巴细胞(TIL)的特异性识别及杀伤功能,有效缓解紫杉醇的毒副作用[38–39]。在近期研究中我们发现红菇多糖可以抑制肿瘤微环境中PD-L1的表达,推测PD-L1是红菇多糖介导免疫调节的重要靶标。Wang等[40]探索发现灵芝多糖处理过的B淋巴细胞PD-1蛋白分泌显著降低,揭示灵芝多糖可以通过可能选择性结合PD-1蛋白,使蛋白质的构象变化并导致PD-1蛋白泛素化和降解,证明了PD-1蛋白是灵芝多糖介导免疫调节的重要靶标。
基于多糖结构及成分等特点我们推测多糖可以直接结合到细胞表面受体,通过下调肿瘤以及淋巴细胞表面免疫负调控因子的表达,并协同化疗药物治疗,增强化疗药物对肿瘤细胞的敏感性,促进机体的免疫应答,增强其对肿瘤的清除率,这也表明食用菌多糖有望从一定程度上逆转肿瘤细胞通过免疫检查抗原引起的免疫逃逸,恢复机体的免疫功能。
2.2 食药用真菌多糖促进主要组织相容性复合体(MHC)类分子表达主要组织相容性复合体(MHC),又称白细胞抗原(HLA)系统,位于免疫功能相关基因最集中、基因密度最高、多态性最丰富、与疾病关联最密切的一个区域,包含了MHC I类分子以及MHC II类分子[41],其主要作用是递呈外源性抗原参与机体的免疫反应。肿瘤抗原只有在与免疫细胞表面MHC类分子结合后才能为免疫细胞所识别和有效杀伤。随着肿瘤细胞的生长与进化,肿瘤细胞可以通过改变MHC类的表达进而减少T细胞识别,因此,通过增强免疫细胞表面MHC和共刺激分子有助于抑制肿瘤细胞免疫逃逸增强淋巴细胞对肿瘤细胞的识别[42]。
Sun等[43]报道了灵芝多糖(GL-PS)对B16F10黑素瘤细胞MHC-I分子和协同刺激因子的影响,利用灵芝多糖处理B16F10黑素瘤细胞后,结果表明B16F10细胞表面MHC I类的受体分子以及B16F10上的B7-1和B7-2蛋白(2个重要的共刺激分子)表达都增强,此外灵芝多糖作用下的B16F10黑素瘤细胞与PHA活化的小鼠脾淋巴细胞共培养,淋巴细胞介导的抗B16F10细胞毒活性较对照组明显提高,表明灵芝多糖通过增强MHC-I共刺激分子的表达有效抑制肿瘤细胞的免疫逃逸。Masuda团队[44]发现灰树花多糖YM-2A组分可以增强树突状细胞MHC II类分子和免疫蛋白CD86的表达以及巨噬细胞MHC II类的表达,同时促进促炎因子CD11b+的表达,诱导全身性抗肿瘤T细胞反应,抑制免疫逃逸达到抑制肿瘤的目的。Pi等[45]发现灵芝多糖具有生物佐剂活性,灵芝多糖PS-F2可以刺激树突状细胞(DC)表达成熟标记分子MHC II和CD40、CD80、CD86以方便T淋巴细胞识别并杀伤肿瘤细胞,抑制肿瘤细胞的免疫逃逸,帮助T细胞识别正常化。
MHC类分子在T细胞自身耐受和形成中都起着至关重要的作用,食药用菌多糖可以通过刺激免疫细胞表面MHC类分子的表达,规避肿瘤细胞通过抗原递呈细胞如巨噬细胞等引起的免疫逃逸途径。
2.3 食药用真菌多糖对肿瘤细胞表面跨膜受体Fas因子的调节作用在肿瘤发生发展过程中,肿瘤细胞进化出许多机制来破坏免疫系统并抑制抗肿瘤免疫反应,其中跨膜受体(Fas)与Fas配体(FasL)在免疫逃逸中起重要作用,因Fas与Fas配体(FasL)交联后可诱导免疫细胞凋亡。肿瘤细胞表面Fas表达明显低下的同时FasL高表达,使进入肿瘤组织周围的免疫细胞,通过肿瘤细胞表达的FasL与免疫细胞表达的Fas结合,激活免疫细胞的凋亡信号途径,导致免疫细胞的凋亡,从而使肿瘤成为机体的免疫豁免部位而逃避免疫系统的攻击[46–47]。目前研究发现,食用菌多糖可通过不同途径对肿瘤细胞Fas/FasL系统进行调控,逆转肿瘤细胞的免疫逃逸。孟运莲等[48]研究发现茯苓多糖能增强淋巴细胞Fas、Bax基因的表达,增加其对自身或其他免疫细胞FasL的凋亡敏感性,并下调肿瘤细胞FasL的表达而降低其对Fas/Fasl系统的反击能力,防止肿瘤细胞发生免疫逃逸,同时辅助细胞毒性T淋巴细胞对肿瘤细胞的杀伤作用。Liang等[49]在研究中指出灵芝多糖(GLP)处理的结肠癌HCT-116细胞导致细胞活力显著降低(P < 0.01),并进一步揭示结肠癌细胞暴露于GLP后其Fas和caspase-3蛋白表达上调,且可以通过促进肿瘤细胞caspase-8蛋白的表达诱导肿瘤细胞凋亡。当Fas/FasL系统出现功能障碍时,活化的T细胞处于异常状态,肿瘤细胞因此能获得免疫逃逸,食用菌多糖可以通过上调肿瘤细胞跨膜受体Fas的表达,调节caspase-3及caspase-9的表达促进肿瘤细胞的凋亡[50]。通过调节Fas/FasL系统来增强淋巴细胞对肿瘤细胞的识别杀伤作用,抑制肿瘤细胞对免疫系统的反攻击,这一思路对进一步拓展肿瘤治疗的思路和方法具有重要价值。
与正常细胞相比,肿瘤细胞具有不同的“糖基化涂层”。由于免疫细胞表达大量的不同类型的糖基化依赖性凝集素受体,所以它们能感知自身环境中糖基化标记的变化并做出相应的反应,而这可能会导致免疫抑制[51]。结合我们课题组对多糖成分以及结构的初步分析,多糖是一种与多肽结合的糖蛋白复合体,这与细胞表面的受体蛋白具有相通之处故推测其影响多种免疫细胞,以及肿瘤细胞,可能是干预肿瘤细胞糖基化来减轻免疫抑制作用。对于具体作用机制还有待更深入的研究。
3 食药用真菌多糖对肿瘤微环境的调节肿瘤的增殖与生长主要由于肿瘤细胞建立的肿瘤微环境,肿瘤微环境包括肿瘤浸润免疫细胞、细胞因子及微环境基质,对机体免疫具有重要调节作用。微环境中存在大量的免疫抑制细胞,它们对免疫具有负向调节作用,促进肿瘤的免疫逃逸[52]。肿瘤微环境内是一个强免疫抑制的环境,微环境和肿瘤细胞之间分泌的免疫抑制因子更是可以通过相互作用,不仅促进肿瘤的生长、转移更影响了肿瘤细胞的免疫逃逸[53]。
3.1 食药用真菌多糖对肿瘤微环境中免疫细胞的调节作用食药用真菌多糖在肿瘤微环境中可以参与免疫调控,研究表明香菇多糖能抑制调节性T细胞(Tregs)的增殖,Th2由于CD3+/CD8+的值及CTL (细胞毒性T淋巴细胞)的升高而转变为Th1,促使微环境中的Th1/Th2恢复平衡,改善微环境的免疫状态、减轻免疫抑制、减少肿瘤细胞的增殖转移及保持正常的免疫应答[54]。蔡庆超等[55]研究发现灵芝多糖可通过调节肿瘤微环境中Treg的水平抑制肿瘤免疫逃逸,达到抑制小鼠肿瘤生长的作用。Kim[56]调查了冬虫夏草多糖对肿瘤微环境中的树突状细胞(DC)的影响。冬虫夏草多糖促进肿瘤微环境中肿瘤细胞及抗原呈递细胞MHC类分子和协同刺激因子如CD40、CD80、CD86、MHC-I和MHC-II分子的表达,增强同种异体T细胞刺激,并减少内吞作用。证明冬虫夏草多糖可以通过TLR4信号传导途径诱导DC成熟,在肿瘤微环境中可以调控抑制性免疫细胞,增强免疫细胞的免疫应答,抑制肿瘤细胞免疫逃逸。在我们的研究中发现灵芝孢子粉多糖显著增加外周血中的细胞毒性T细胞(Tc)和辅助T细胞(Th)的数量,调节肿瘤微环境中Tregs的数量,恢复肿瘤微环境中T淋巴细胞对肿瘤细胞的监视及杀伤的作用,抑制肿瘤小鼠体内肿瘤生长[38]。近期发现灰树花多糖能抑制肿瘤微环境中Tregs增殖,促进CD8+细胞浸润到肿瘤微环境中,调节肿瘤微环境免疫状态,促进肿瘤细胞凋亡。
在肿瘤微环境中Tregs可以抑制CD8+等免疫细胞激活从而有利于肿瘤免疫逃逸的发生。食药用菌多糖可以通过调节Tregs,激活CD8+细胞改善肿瘤微环境免疫状态,抑制肿瘤免疫逃逸,进而抑制肿瘤生长。
3.2 食药用真菌多糖对肿瘤微环境中肿瘤细胞分泌免疫抑制因子的调节作用肿瘤细胞可产生多种免疫抑制分子,抑制免疫细胞的功能,逃避机体免疫系统的攻击。白细胞介素10 (IL-10)、转化生长因子β1 (TGF-β1)和血管内皮生长因子(vascular endothelialgrowth factor,VEGF)为肿瘤细胞中常见的免疫抑制分子[57],肿瘤免疫疗法的最重要目标之一是对抗抑制性免疫细胞的这种负调控作用。Liu等[58]研究报道香菇多糖联合化疗药物治疗恶性肿瘤时,香菇多糖可通过降低肿瘤细胞分泌免疫抑制因子IL-6、TNF-α和TGF-β的表达来调节Treg淋巴细胞的平衡,抑制肿瘤细胞的免疫逃逸,从而增强顺铂化疗药物的抗肿瘤作用增强其对肿瘤细胞的细胞毒性作用。灵芝多糖[59]、猴头菇多糖[59]等均可通过调节细胞因子(IFN-γ、TNF-α、IL-2)分泌水平、增强机体免疫功能的作用减轻肿瘤发生过程中的免疫抑制作用。蛹虫草多糖可以明显增加肿瘤微环境中巨噬细胞的典型促炎细胞因子(NO、IL-1β、TNF-α和IL-6)且降低免疫抑制细胞因子IL-10和TGF-β的mRNA水平[60],我们实验结果中发现灵芝孢子粉多糖除了能抑制肿瘤细胞以及T淋巴细胞表面免疫负调控因子的表达,还能抑制B淋巴细胞免疫负调控因子的分泌,抑制肿瘤血管生成因子的分泌[61]。肿瘤细胞分泌免疫抑制因子是肿瘤免疫逃逸的重要途径,食用菌多糖可在一定程度上拮抗肿瘤细胞分泌的免疫抑制因子,阻断其发挥作用的免疫抑制通路,增强抗肿瘤免疫反应。
肿瘤微环境的重建引起了机体病理生理反应。这个重建的独特网络可以利用免疫细胞的相互作用来支持肿瘤的代谢和生长。食药用真菌多糖可以靶向肿瘤微环境、调节免疫应答、降低免疫抑制细胞对于免疫细胞的负调控作用从而起到抗肿瘤作用。
4 讨论肿瘤免疫逃逸是肿瘤形成必不可少的特征之一,通过肿瘤细胞自身的修饰和肿瘤微环境的改变而发生,也正因为各种肿瘤免疫逃逸机制处于一个复杂的免疫网络中,所以即便是目前常用来治疗癌症的免疫疗法包括研究热门免疫检查点疗法效果也因此受到限制[62]。与直接治疗的免疫抑制剂相比,食药用真菌多糖具有作用靶点多而全面的优势。因为糖类自身的结构以及性质让以糖类为基础的药物作用靶点在细胞表面[63],因此食药用真菌多糖可靶向定位肿瘤微环境,破坏肿瘤微环境形成的条件,多靶点靶向调节肿瘤细胞表面特异性识别抗原。拮抗肿瘤细胞分泌的免疫抑制因子,活化免疫细胞,解除肿瘤微环境中肿瘤细胞对巨噬细胞、NK细胞、T淋巴细胞、B淋巴细胞以及DC细胞的抑制,恢复免疫系统功能,识别和清除肿瘤细胞(图 2)。基于肿瘤免疫微环境的差异性,未来的肿瘤免疫治疗可以加强对肿瘤、肿瘤微环境和肿瘤免疫逃逸间相互作用的研究,弥补单一的免疫疗法存在的缺陷。近期研究中我们通过实验观察灰树花多糖、红菇多糖对4T1乳腺癌移植鼠肿瘤微环境中PD-L1和CTLA-4表达的影响中已被证实,食药用真菌多糖能抑制肿瘤细胞内免疫下调因子PD-L1和CTLA-4的表达水平,在与化疗药物协同作用时,能在不同环节更好地调控调节性T细胞的作用,在消除肿瘤微环境的局部免疫耐受方面,具有一定的协同治疗优势。因此,深入探索食药用菌多糖与肿瘤表面受体靶向选择性等特点,探索其与化疗药物和其他免疫疗法的联合作用对于肿瘤治愈率是否提高是一个潜在的研究方向。

|
| 图 2 食药用真菌多糖调节肿瘤免疫逃逸机制 Figure 2 Edible and medicinal fungal polysaccharide regulate tumor immune escape mechanism. |
然而,由于食药用真菌多糖组分中单糖的种类繁多,连接方式千差万别,导致多糖的结构极其复杂,构效关系没有固定规律,因此给研究带来了一定困难。尽管食药用真菌多糖在抗肿瘤免疫逃逸还存在以下问题:食药用真菌多糖对于肿瘤微环境的调控基因仍有待研究,食药用菌多糖具体与肿瘤细胞以及免疫细胞结合原理以及机制尚不清楚,对食药用真菌多糖的共同特性研究分类尚不明确。但随着现代肿瘤免疫学以及分子生物学的发展和多糖作用机制研究的深入,借助先进技术对多糖进行研究和开发,未来食药用真菌多糖的研究具有极大的开发潜力,期望让食药用真菌多糖在肿瘤治疗中免疫整体调节的本质将会得到进一步揭示,让有着广泛应用前景的食药用真菌多糖类肿瘤免疫逃逸调节剂的研发进一步为患者带来福音。
| [1] | Vasquez L, Castro D, León J, Beltrán B. Inmunoterapia en cáncer: de los inicios al premio Nobel. Revista Peruana De Medicina Experimental Y Salud Publica, 2020, 37(1): 115-121. DOI:10.17843/rpmesp.2020.371.4329 |
| [2] | Li MX, Luo T, Huang Y, Su JY, Li D, Chen XH, Zhang YF, Huang LH, Li SX, Jiao CW, Li WZ, Xie YZ, Li WD. Polysaccharide from Pycnoporus sanguineus ameliorates dextran sulfate sodium-induced colitis via helper T cells repertoire modulation and autophagy suppression. Phytotherapy Research, 2020, 34(10): 2649-2664. DOI:10.1002/ptr.6695 |
| [3] | Li XM, Xie YZ, Yang BB. Characterizing novel anti-oncogenic triterpenoids from ganoderma. Cell Cycle, 2018, 17(5): 527-528. DOI:10.1080/15384101.2017.1315493 |
| [4] | Li XM, Wu QP, Xie YZ, Ding YR, Du WW, Sdiri M, Yang BB. Ergosterol purified from medicinal mushroom Amauroderma rude inhibits cancer growth in vitro and in vivo by up-regulating multiple tumor suppressors. Oncotarget, 2015, 6(19): 17832-17846. DOI:10.18632/oncotarget.4026 |
| [5] | 李向敏. 皱盖假芝和灵芝孢子中甾醇类化合物抗肿瘤作用机制研究. 华南理工大学博士学位论文, 2015. |
| [6] |
Fu YJ, Gao X, Tian Z, Li RJ, Wang MX, Tian JX, Zhang BH, Yang LP. Recearch progress on immunomodulatory effects of lentinan on tumor microenvironment. Drugs & Clinic, 2019, 34(9): 2870-2875.
(in Chinese) 符映均, 高欣, 田振, 李儒杰, 王明莃, 田佳鑫, 张碧华, 杨莉萍. 香菇多糖对肿瘤微环境免疫调节作用的研究进展. 现代药物与临床, 2019, 34(9): 2870-2875. |
| [7] |
Wu LJ, Qi M, Li N, Lei YH, Zhang DM, Chen JX. Curative efficacy of lentinan injection combined with CHOP chemotherapy in the treatment of diffuse large B cell lymphoma and its effects on the Bcl-6, Ki-67, VEGF, β2-MG expressions. Progress in Modern Biomedicine, 2018, 18(11): 2122-2126, 2149. (in Chinese) 吴必嘉, 龚峻梅, 陈娟娟, 黄斯勇, 高飞. 香菇多糖注射液联合CHOP化疗治疗弥漫性大B细胞淋巴瘤的疗效及对Bcl-6、Ki-67、VEGF、β2-MG表达的影响. 现代生物医学进展, 2018, 18(11): 2122-2126, 2149. |
| [8] | Deng BJ, Gong JM, Chen JJ, Huang SY, Gao F. Natural products and their derivatives: promising modulators of tumor immunotherapy. Journal of Leukocyte Biology, 2020, 108(2): 493-508. DOI:10.1002/JLB.3MR0320-444R |
| [9] |
Wang YX, Han JG. Pay attention to the therapeutic effect of ganoderma sinense polysaccharide tablets on tumor China. Health Care and Nutrition, 2013(9): 5343.
(in Chinese) 王永霞, 韩金刚. 重视紫芝多糖片对肿瘤的治疗作用. 中国保健营养, 2013(9): 5343. |
| [10] |
Liu M, Ju CY, Liu Y, Xiao XJ, Zhang LM, Sun W, Zhu YM, Li L, Zhu YL. The efficacy and safety of Ganoderma lucidum polysaccharides in the treatment of depression. Capital Food Medicine, 2016, 23(12): 53-55.
(in Chinese) 刘勐, 居春阳, 刘羽, 肖兴军, 张丽梅, 孙威, 朱延梅, 李磊, 朱雨岚. 灵孢多糖注射液联合西酞普兰治疗抑郁症的疗效和安全性研究. 首都食品与医药, 2016, 23(12): 53-55. |
| [11] | Cör D, Knez Ž, Knez Hrnčič M. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: a review. Molecules, 2018, 23(3): 649. DOI:10.3390/molecules23030649 |
| [12] |
Yang DY, Jiao Y, Du ZJ, Hu XJ, Liu F. Therapeutic effects of polyporus polysaccharide injection combined with cisplatin intraperitoneal injection on seroperitoneum due to gastric cancer. Hebei Medical Journal, 2018, 40(5): 725-727, 731.
(in Chinese) 杨冬野, 焦洋, 杜志坚, 胡晓杰, 刘飞. 猪苓多糖注射液联合顺铂腹腔给药治疗胃癌腹腔积液患者的疗效观察. 河北医药, 2018, 40(5): 725-727, 731. |
| [13] |
Li XQ, Zhang BB, Zhang MK, Song JW, Wang XL. Coriolus versicolor polysaccharide combined with XELOX regimen in the treatment of advanced colorectal cancer. World Latest Medicine Information, 2019, 19(99): 230, 235.
(in Chinese) 李秀芹, 张斌斌, 张明奎, 宋家伟, 王雪莲. 云芝多糖联合XELOX方案在晚期大肠癌治疗中的疗效观察. 世界最新医学信息文摘, 2019, 19(99): 230, 235. |
| [14] |
Pang LF. Clinical study about parenteral solution of Tremella polysaccharide improving quality of life of patients with liver cancer during chemotherapy. Journal of Hubei University of Chinese Medicine, 2014, 16(4): 85-86.
(in Chinese) 庞良芳. 银耳孢糖提高肝癌化疗患者生存质量的研究. 湖北中医药大学学报, 2014, 16(4): 85-86. DOI:10.3969/j.issn.1008-987x.2014.04.31 |
| [15] | 史春雨. 复方茯苓多糖口服液抗肿瘤作用机制研究. 南方医科大学硕士学位论文, 2018. |
| [16] | Mao GH, Zhang ZH, Fei F, Ding YY, Zhang WJ, Chen H, Ali SS, Zhao T, Feng WW, Wu XY, Yang LQ. Effect of Grifola frondosa polysaccharide on anti-tumor activity in combination with 5-Fu in Heps-bearing mice. International Journal of Biological Macromolecules, 2019, 121: 930-935. DOI:10.1016/j.ijbiomac.2018.10.073 |
| [17] | Jiang XJ, Wang J, Deng XY, Xiong F, Ge JS, Xiang B, Wu X, Ma J, Zhou M, Li XL, Li Y, Li GY, Xiong W, Guo C, Zeng ZY. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Molecular Cancer, 2019, 18(1): 10. DOI:10.1186/s12943-018-0928-4 |
| [18] | Wang YA, Li XL, Mo YZ, Fan CM, Tang L, Xiong F, Guo C, Xiang B, Zhou M, Ma J, Huang X, Wu X, Li Y, Li GY, Zeng ZY, Xiong W. Effects of tumor metabolic microenvironment on regulatory T cells. Molecular Cancer, 2018, 17(1): 168. DOI:10.1186/s12943-018-0913-y |
| [19] |
Wen MJ, Yang S, Wang QH, Wen CL, Huang XF, Wang X, Zhou PH. Targeting immune checkpoints to treat cancer-The 2018 Nobel prize in physiology or medicine. Progress in Physiological Sciences, 2019, 50(4): 315-321.
(in Chinese) 温铭杰, 杨硕, 王惊华, 韦春莲, 黄雪峰, 王玺, 周鹏辉. 癌症治疗的新支柱-免疫检查点疗法——2018年诺贝尔生理学或医学奖. 生理科学进展, 2019, 50(4): 315-321. DOI:10.3969/j.issn.0559-7765.2019.04.017 |
| [20] |
Hu M, Liu QY. Key factors limiting the efficacy of immune-checkpoint blockade and research progress on combined anti-tumor strategies. Chinese Journal of Cancer Biotherapy, 2019, 26(9): 933-940.
(in Chinese) 胡淼, 刘秋燕. 限制免疫检查点阻断疗效的关键因素及联合抗肿瘤对策的研究进展. 中国肿瘤生物治疗杂志, 2019, 26(9): 933-940. |
| [21] | Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer-clinical challenges and opportunities. Nature Reviews Clinical Oncology, 2020, 17(9): 527-540. DOI:10.1038/s41571-020-0363-5 |
| [22] | Wang QT, Nie Y, Sun SN, Lin T, Han RJ, Jiang J, Li Z, Li JQ, Xiao YP, Fan YY, Yuan XH, Zhang H, Zhao BB, Zeng M, Li SY, Liao HX, Zhang J, He YW. Tumor-associated antigen-based personalized dendritic cell vaccine in solid tumor patients. Cancer Immunology, Immunotherapy, 2020, 69(7): 1375-1387. DOI:10.1007/s00262-020-02496-w |
| [23] | Darapu H, Li P, Paluri R. SUN-332 autoimmune endocrinopathies associated with CTLA-4 inhibitors: a meta-analysis. Journal of the Endocrine Society, 2019, 3(S1): SUN-332. |
| [24] | Sanchez-Paulete AR, Labiano S, Rodriguez-Ruiz ME, Azpilikueta A, Etxeberria I, Bolaños E, Lang V, Rodriguez M, Aznar MA, Jure-Kunkel M, Melero I. Deciphering CD137(4-1BB) signaling in T-cell costimulation for translation into successful cancer immunotherapy. European Journal of Immunology, 2016, 46(3): 513-522. DOI:10.1002/eji.201445388 |
| [25] | Mastracci L, Fontana V, Queirolo P, Carosio R, Grillo F, Morabito A, Banelli B, Tanda E, Boutros A, Dozin B, Gualco M, Salvi S, Romani M, Spagnolo F, Poggi A, Pistillo MP. Response to ipilimumab therapy in metastatic melanoma patients: potential relevance of CTLA-4+ tumor infiltrating lymphocytes and their in situ localization. Cancer Immunology, Immunotherapy, 2020, 69(4): 653-662. DOI:10.1007/s00262-020-02494-y |
| [26] | Fujii R, Friedman ER, Richards J, Tsang KY, Heery CR, Schlom J, Hodge JW. Enhanced killing of chordoma cells by antibody-dependent cell-mediated cytotoxicity employing the novel anti-PD-L1 antibody avelumab. Oncotarget, 2016, 7(23): 33498-33511. DOI:10.18632/oncotarget.9256 |
| [27] | Grenga I, Donahue RN, Lepone LM, Richards J, Schlom J. A fully human IgG1 anti-PD-L1 MAb in an in vitro assay enhances antigen-specific T-cell responses. Clinical & Translational Immunology, 2016, 5(5): e83. |
| [28] | Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized functions in immune regulation. Immunity, 2016, 44(5): 989-1004. DOI:10.1016/j.immuni.2016.05.001 |
| [29] | Kang C, Syed YY. Atezolizumab (in combination with Nab-paclitaxel): a review in advanced triple-negative breast cancer. Drugs, 2020, 80(6): 601-607. DOI:10.1007/s40265-020-01295-y |
| [30] | Schmid P, Cruz C, Braiteh FS, Eder JP, Tolaney S, Kuter I, Nanda R, Chung C, Cassier P, Delord JP, Gordon M, Li YJ, Liu B, O'Hear C, Fasso M, Molinero L, Emens LA. Abstract 2986:Atezolizumab in metastatic TNBC (mTNBC): long-term clinical outcomes and biomarker analyses. Cancer Research, 2017, 77(S13): 2986-2986. |
| [31] | Jhaveri K, Wang R, Teplinsky E, Chandarlapaty S, Solit D, Cadoo K, Speyer J, D'Andrea G, Adams S, Patil S, Haque S, O'Neill T, Friedman K, Esteva FJ, Hudis C, Modi S. A phase I trial of ganetespib in combination with paclitaxel and trastuzumab in patients with human epidermal growth factor receptor-2(HER2)-positive metastatic breast cancer. Breast Cancer Research, 2017, 19(1): 89. DOI:10.1186/s13058-017-0879-5 |
| [32] | Kok M, Voorwerk L, Horlings H, Sikorska K, Van Der Vijver K, Slagter M, Warren S, Ong S, Wiersma T, Russell N, Lalezari F, de Maaker M, Kemper I, Mandjes IA, Chalabi M, Sonke GS, Salgado R, Linn SC, Schumacher T, Blank CU. Adaptive phase II randomized trial of nivolumab after induction treatment in triple negative breast cancer (TONIC trial): final response data stage I and first translational data. American Society of Clinical Oncology, 2018, 36(S15): 1012. |
| [33] | Wang J, Sanmamed MF, Datar I, Su TT, Ji L, Sun JW, Chen L, Chen YS, Zhu GF, Yin WW, Zheng LH, Zhou T, Badri T, Yao S, Zhu S, Boto A, Sznol M, Melero I, Vignali DAA, Schalper K, Chen LP. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell, 2019, 176(1/2): 334-347.e12. |
| [34] | Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nature Reviews Clinical Oncology, 2016, 13(5): 273-290. DOI:10.1038/nrclinonc.2016.25 |
| [35] | Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity, 1999, 11(2): 141-151. DOI:10.1016/S1074-7613(00)80089-8 |
| [36] | Chen LP, Ashe S, Brady WA, Hellström I, Hellström KE, Ledbetter JA, McGowan P, Linsley PS. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell, 1992, 71(7): 1093-1102. DOI:10.1016/S0092-8674(05)80059-5 |
| [37] | Ina H, Yoneda M, Kanda M, Kodera Y, Kabeya M, Yuasa S, Kataoka T, Furuta R, Ina K. Lentinan, a shiitake mushroom β-glucan, stimulates tumor-specific adaptive immunity through PD-L1 down-regulation in gastric cancer cells. Medicinal Chemistry, 2016, 6(12): 710-714. |
| [38] | Su JY, Su L, Li D, Shuai O, Zhang YF, Liang HJ, Jiao CW, Xu ZC, Lai Y, Xie YZ. Antitumor activity of extract from the sporoderm-breaking spore of Ganoderma lucidum: restoration on exhausted cytotoxic T cell with gut microbiota remodeling. Frontiers in Immunology, 2018, 9: 1765. DOI:10.3389/fimmu.2018.01765 |
| [39] | Su JY, Li D, Chen QJ, Li MX, Su L, Luo T, Liang DL, Lai GX, Shuai O, Jiao CW, Wu QP, Xie YZ, Zhou XX. Corrigendum: anti-breast cancer enhancement of a polysaccharide from spore of Ganoderma lucidum with paclitaxel: suppression on tumor metabolism with gut microbiota reshaping. Frontiers in Immunology, 2019, 10: 1224. DOI:10.3389/fimmu.2019.01224 |
| [40] | Wang G, Wang L, Zhou JL, Xu XX. The possible role of PD-1 protein in Ganoderma lucidum-mediated immunomodulation and cancer treatment. Integrative Cancer Therapies, 2019, 18: 1-13. DOI:10.1177/1534735419880275 |
| [41] |
Fan LA, Yan WH. Non-classical MHC class I molecules and immune regulation. Shanghai Journal of Immunology, 2003, 23(5): 289-292.
(in Chinese) 范丽安, 颜卫华. 非经典MHC I类分子与免疫调节. 上海免疫学杂志, 2003, 23(5): 289-292. DOI:10.3969/j.issn.1001-2478.2003.05.001 |
| [42] | 骆亚莉, 刘永琦, 安方玉, 张利英, 李亚玲, 颜春鲁, 冯彩琴, 李研. 肿瘤免疫逃逸机制研究进展//第十三届全国免疫学学术大会摘要汇编. 上海: 中国免疫学会, 2018. |
| [43] | Sun LX, Lin ZB, Duan XS, Lu J, Ge ZH, Li XF, Li XJ, Li M, Xing EH, Song YX, Jia J, Li WD. Enhanced MHC class I and costimulatory molecules on B16F10 cells by Ganoderma lucidum polysaccharides. Journal of Drug Targeting, 2012, 20(7): 582-592. DOI:10.3109/1061186X.2012.697167 |
| [44] | Masuda Y, Nakayama Y, Tanaka A, Naito K, Konishi M. Antitumor activity of orally administered maitake α-glucan by stimulating antitumor immune response in murine tumor. PLoS ONE, 2017, 12(3): e0173621. DOI:10.1371/journal.pone.0173621 |
| [45] | Pi CC, Chu CL, Lu CY, Zhuang YJ, Wang CL, Yu YH, Wang HY, Lin CC, Chen CJ. Polysaccharides from Ganoderma formosanum function as a Th1 adjuvant and stimulate cytotoxic T cell response in vivo. Vaccine, 2014, 32(3): 401-408. DOI:10.1016/j.vaccine.2013.11.027 |
| [46] | Zhang BL, Sun T, Xue LY, Han XH, Zhang BL, Lu N, Shi YK, Tan W, Zhou YF, Zhao D, Zhang XM, Guo YL, Lin DX. Functional polymorphisms in FAS and FASL contribute to increased apoptosis of tumor infiltration lymphocytes and risk of breast cancer. Carcinogenesis, 2007, 28(5): 1067-1073. |
| [47] | O'Brien DI, Nally K, Kelly RG, O'Connor TM, Shanahan F, O'Connell J. Targeting the Fas/Fas ligand pathway in cancer. Expert Opinion on Therapeutic Targets, 2005, 9(5): 1031-1044. DOI:10.1517/14728222.9.5.1031 |
| [48] |
Meng YL, Cai LH, Wu HF, Zhang LN. Immunohistochemical observation on the anti-tumor effect of the Pachyman polysaccharides modified by chemical technique. Medical Journal of Wuhan University, 2007, 28(1): 67-69.
(in Chinese) 孟运莲, 蔡丽华, 吴慧芬, 张俐娜. 化学修饰的茯苓多糖抗肿瘤效应的免疫组织化学观察. 武汉大学学报(医学版), 2007, 28(1): 67-69. |
| [49] | Liang ZEN, Guo YT, Yi YJ, Wang RC, Hu QL, Xiong XY. Ganoderma lucidum polysaccharides target a Fas/caspase dependent pathway to induce apoptosis in human colon cancer cells. Asian Pacific Journal of Cancer Prevention, 2014, 15(9): 3981-3986. DOI:10.7314/APJCP.2014.15.9.3981 |
| [50] | Jiao CW, Chen W, Tan XP, Liang HJ, Li JY, Yun H, He CY, Chen JM, Ma XW, Xie YZ, Yang BB. Ganoderma lucidum spore oil induces apoptosis of breast cancer cells in vitro and in vivo by activating caspase-3 and caspase-9. Journal of Ethnopharmacology, 2020, 247: 112256. DOI:10.1016/j.jep.2019.112256 |
| [51] | RodrÍguez E, Schetters STT, Van Kooyk Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nature Reviews Immunology, 2018, 18(3): 204-211. DOI:10.1038/nri.2018.3 |
| [52] | Thomas PD, Kahn M. Kat3 coactivators in somatic stem cells and cancer stem cells: biological roles, evolution, and pharmacologic manipulation. Cell Biology and Toxicology, 2016, 32(1): 61-81. DOI:10.1007/s10565-016-9318-0 |
| [53] | Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends in Immunology, 2016, 37(3): 208-220. DOI:10.1016/j.it.2016.01.004 |
| [54] | Wang XE, Wang YH, Zhou Q, Peng M, Zhang J, Chen M, Ma LJ, Xie GM. Immunomodulatory effect of lentinan on aberrant T subsets and cytokines profile in non-small cell lung cancer patients. Pathology & Oncology Research, 2020, 26(1): 499-505. DOI:10.1007/s12253-018-0545-y |
| [55] |
Cao QC, Lin ZB, Li WD. Ganoderma lucidum polysaccharide peptide inhibits the growth of mouse LLC implanted tumors by regulating the level of Treg in the tumor microenvironment. Chinese Pharmacological Bulletin, 2015, 31(B11): 220-221.
(in Chinese) 蔡庆超, 林志彬, 李卫东. 灵芝多糖肽通过调节肿瘤微环境中Treg水平抑制小鼠LLC种植瘤生长. 中国药理学通报, 2015, 31(B11): 220-221. |
| [56] | Kim HS, Kim JY, Kang JS, Kim HM, Kim YO, Hong IP, Lee MK, Hong JT, Kim Y, Han SB. Cordlan polysaccharide isolated from mushroom Cordyceps militaris induces dendritic cell maturation through toll-like receptor 4 signalings. Food and Chemical Toxicology, 2010, 48(7): 1926-1933. DOI:10.1016/j.fct.2010.04.036 |
| [57] |
Lin ZB. Antitumor effect of Ganoderma (Lingzhi) mediated by immunological mechanism and its clinical application. Chinese Journal of Pharmacology and Toxicology, 2015, 29(6): 865-882.
(in Chinese) 林志彬. 灵芝抗肿瘤作用的免疫学机制及其临床应用. 中国药理学与毒理学杂志, 2015, 29(6): 865-882. DOI:10.3867/j.issn.1000-3002.2015.06.001 |
| [58] | Liu XD, Li M, Li WX, Wang QY, Zhang HX. Combined effect of lentinan and cisplatin on cytokines IL-6, TNF-α, and TGF-β in tumor therapy. International Journal of Polymer Science, 2019, 2019: 4064703. |
| [59] | Sun LX, Lin ZB, Li XJ, Li M, Lu J, Duan XS, Ge ZH, Song YX, Xing EH, Li WD. Promoting effects of Ganoderma lucidum polysaccharides on B16F10 cells to activate lymphocytes. Basic & Clinical Pharmacology & Toxicology, 2011, 108(3): 149-154. |
| [60] | Bi SX, Huang WJ, Chen S, Huang CH, Li CL, Guo ZY, Yang JN, Zhu JH, Song LY, Yu RM. Cordyceps militaris polysaccharide converts immunosuppressive macrophages into M1-like phenotype and activates T lymphocytes by inhibiting the PD-L1/PD-1 axis between TAMs and T lymphocytes. International Journal of Biological Macromolecules, 2020, 150: 261-280. DOI:10.1016/j.ijbiomac.2020.02.050 |
| [61] | Chen SD, Yong TQ, Zhang YF, Su JY, Jiao CW, Xie YZ. Anti-tumor and anti-angiogenic ergosterols from Ganoderma lucidum. Frontiers in Chemistry, 2017, 5: 85. DOI:10.3389/fchem.2017.00085 |
| [62] | Lu X, Horner JW, Paul E, Shang XY, Troncoso P, Deng PN, Jiang S, Chang Q, Spring DJ, Sharma P, Zebala JA, Maeda DY, Wang YA, De Pinho RA. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature, 2017, 543(7647): 728-732. DOI:10.1038/nature21676 |
| [63] | 陈国荣. 糖化学基础. 上海: 华东理工大学出版社, 2009. |
 2021, Vol. 61
2021, Vol. 61